


I recently travelled to Japan, where I met the original inventor of our technology. His name is Mr. Tetsuhiko Fujisato, a professional focused on water treatment, public health, and environmental protection.
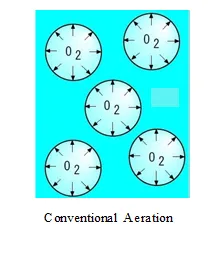
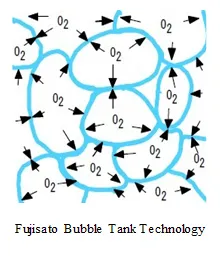
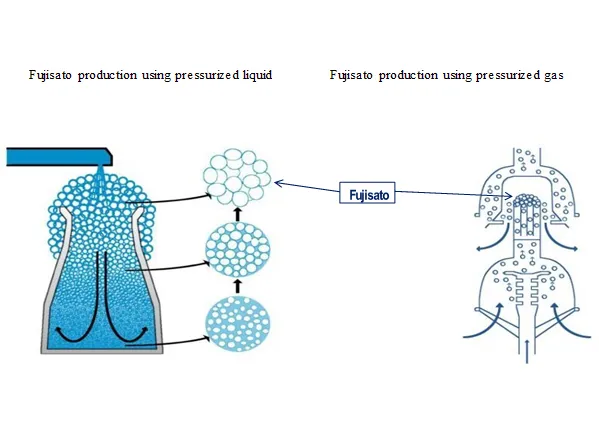

The technology of the saturated water generator instantly transforms the water to be treated into a film, generating saturated water.
The device also generates water with a saturation level that corresponds to the air pressure at the location where it is placed.
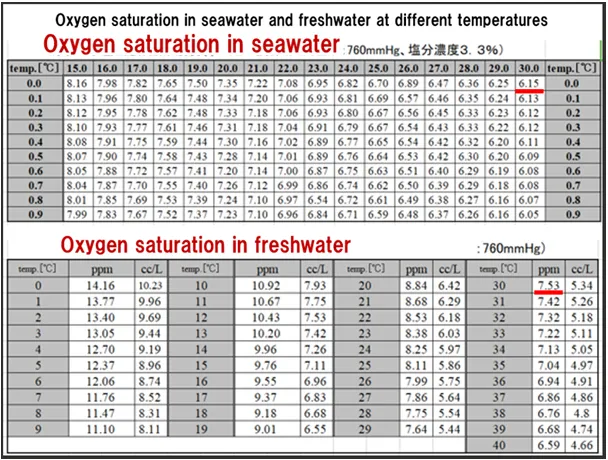
Based on the oxygen saturation concentration table for seawater and freshwater above, a DO concentration of 6.35 mg/L at a water temperature of 31.7°C is wastewater that contains a lot of organic matter and minerals, and is therefore thought to have properties similar to seawater.

By treating the treated water in a film form, even if there is an excess of N2, it will return to the blue N2 saturation line, and even if the O2 content is 0 mg/L, it will automatically return to the yellow O2 saturation line.
It can also be easily operated in deep water.

Super saturated N2 gas and CO2 gas are measured. You must remove those gases to make a room for new air to come in.
| Dissolved concentration of gases (mg/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Measured in ML (a) | Saturation level (b) | a/b | |||||||
| N₂ | 48.6 | 17.8 | 2.7 | ||||||
| CO₂ | 79.5 | 0.58 | 132 | ||||||
| O₂ | 0.71 | 9.56 | 0.07 | ||||||



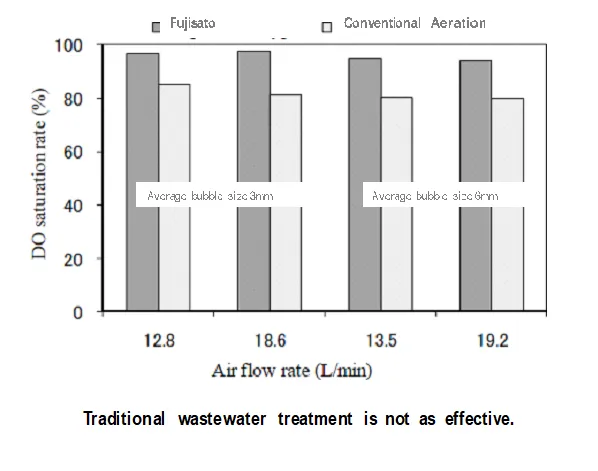
The DO increases by just placing the Fujisato units at the surface of aeration basins. Doing this increased the DO level from 0.3ppm to 3.6ppm. No other change is made, no chemicals, nothing.
Fujisato enables shallow depth aeration. Unlike fine bubble diffusers, Fujisato devices DO NOT require aeration the aeration point to be 14ft (4m) or deeper. 3-4ft (1m) deep water is enough to achieve higher DO and it uses less power.
The Fujisato system works for various applications including wastewater, drinking water, dam/pond/storm water, aquaculture, hydroponics, bio-reactors, algae growth, gas dissolution/stripping etc.

Dissolved Oxygen (DO): Why It Matters in Water Quality, Drinking Water, Aquaculture, and Wastewater Treatment


Dissolved oxygen (DO) refers to the amount of oxygen gas (O₂) mixed into and available within water. While water molecules (H₂O) contain oxygen, aquatic organisms and microbes cannot use that oxygen. They rely on free O₂ molecules dissolved in water for respiration and survival.
| Process / Use | Good DO Level | Purpose |
|---|---|---|
| Wastewater aerobic treatment | 2–4 mg/L | Supports aerobic bacteria, reduces BOD/COD |
| Nitrification | 2 mg/L | Converts ammonia to nitrate |
| Drinking water distribution | 6–8 mg/L | Maintains taste, reduces corrosion |
| Rivers/lakes | 8–12 mg/L | Healthy aquatic habitat |


| DO Level | Dominant Processes | Effect on pH |
|---|---|---|
| High DO | Aerobic respiration, CO2 removal, photosynthesis | pH stable or slightly higher |
| Low DO | Anaerobic activity, CO2 build up, organic acid production, reducing conditions | pH typically decreases |
| Very low / anoxic | Strong anaerobic digestion, H2S formation, acid production | pH drops even further |
Dissolved oxygen is vital for water quality, treatment effectiveness, aquatic health, and overall ecosystem stability. While not directly regulated in drinking water, it is a key indicator of water system health and operational efficiency. Maintaining proper DO is one of the simplest and most effective ways to ensure safe, high-quality water for people, aquatic life, and the environment.

Puroxi Pure Water Global Inc was shortlisted for the awards category, Water Solutions Provider of the Year! As highlighted, it was some good news. I can confirm you that you have been chosen as the 2023/24 winners. Congratulations Zak! The generic winner’s logo has been attached which you can utilize to promote the recognition and the official press release will be conducted in March (defined date to come